PHOTOELECTRON DIFFRACTION FROM FIXED-IN-SPACE MOLECULES OF
HYDROCARBONS (C2H2 AND C2H4)
T.U. Osipov1, C.L. Cocke1, R. Doerner2,
A.L. Landers3, T. Webber4, O. Jagutski4,
A. Demian-Braeuning4, H. Braeuning5, M.H. Prior6,
H. Schmidt-Boecking4 and A. Cassimi7
1) Department of Physics, Kansas State University, Manhattan, KS 66506
2) Institut fur Kernphysik, Universitat Freiburg, Germany
3) Department of Physics, Western Michigan University, Kalamazoo, MI 49008
4) Institut fur Kernphysik, Universitat Frankfurt, Germany
5) Institut fur Kernphysik, Universitat Giessen, Germany
6) Lawrence Berkeley National Laboratory, Berkeley, CA 94720
7) CIRIL/CEA/CNRS, Caen, France
Free molecules of
acetylene (C2H2) and
ethane (C2H4)
have been photo-ionized by X-rays
just above the carbon K-edge. This photoionization is followed with high probability by an
Auger decay and subsequent molecular dissociation. Using a momentum-imaging technique, the
momenta of the photoelectron and all charged molecular fragments were measured in coincidence.
The photoelectron angular distribution, as a function of molecular orientation with respect to
the polarization axis, was then obtained. The photoelectron yield, as a function of X-ray energy,
shows the presence of a shape resonance around 15-20 eV above the ionization potential of the carbon
K-shell electron. From this the complex amplitudes of the partial waves describing the photoelectron
outgoing wave were obtained. These amplitudes can be used to provide information about the molecular
potential in which the photoelectron moves.
Presented analysis resolves the ongoing debate about the presence of the f-wave resonance in
hydrocarbons [1].
Figures:
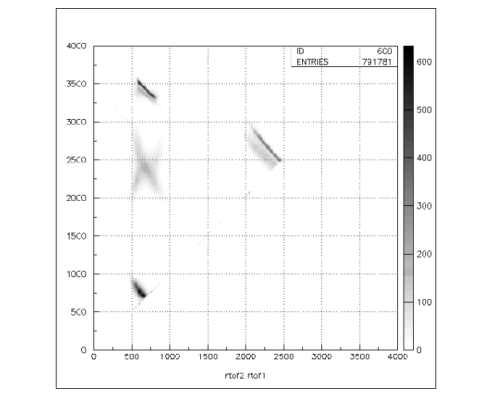
Figure 1:
PIPICO specter: time-of-flight for the first recoil versus time-of-flight for the second
recoil of the C2H2 molecule breakup. Different breakup channels of the molecule are clearly shown.
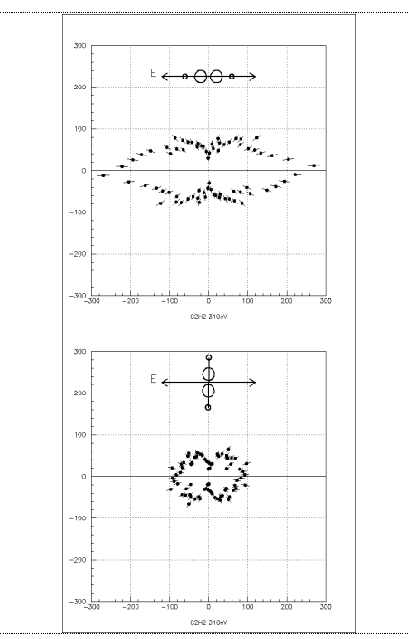
Figure 2:
Angular distribution of 20eV K-photoelectrons from
C2H2 by linearly polarized light.
The plane of the figure is perpendicular to the light propagation. The orientation of
the molecule with respect to the polarization axis is shown on the top of each picture.
References:
1) B. Kempgens et al. Phys. Rev. Lett. 79, 35-38 (1997).
This work was supported by the
Chemical Sciences, Geosciences and Biosciences Division,
Office of Basic Energy Sciences,
Office of Science,
U.S. Department of Energy.
Submitted to ICPEAC 2001, July 2001 in Santa Fe, NM.
This abstract is also available in
Postscript or
Adobe Acrobat formats.

